101.7 FM
88.1HD2
 8.85 c
8.85 c101.7 FM
88.1HD2
Pfizer Canada says it will ask Health Canada to authorize COVID-19 vaccine to extend to children between 12 and 15 years old
BY , Mar 31, 2021 5:31 PM - REPORT AN ERROR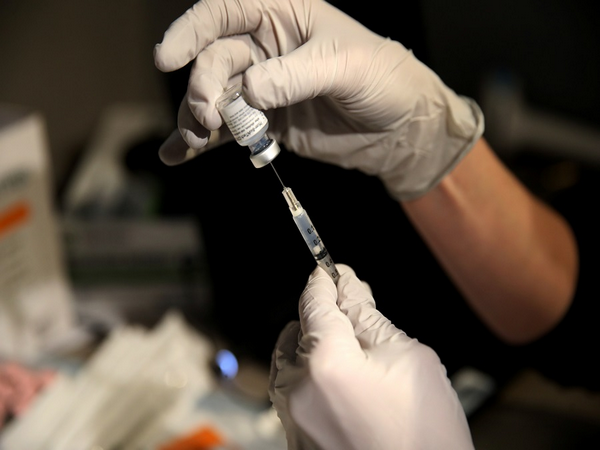
Pfizer Canada says it will be asking Health Canada to amend the authorization for its COVID-19 vaccine to extend to children between 12 and 15 years old. (Photo -ANI)
Pfizer Canada says it will be asking Health Canada to amend the authorization for its COVID-19 vaccine to extend to children between 12 and 15 years old.
The Pfizer-BioNTech vaccine has already been approved for people as young as 16.
The initial clinical trials didn't include younger adolescents, but a follow-up trial in 2,260 kids 12 to 15 in the U.S. has been running since the fall.
The company released preliminary data from that trial Wednesday, saying none of the kids who got the vaccine developed a COVID-19 infection, compared to 18 infections among the kids who were given a placebo.
Experts see this as a step toward possibly beginning shots in this age group before they head back to school in the fall.
Share on
Related News
Sign up for the newsletter
We'll deliver best of entertainment right into your inboxWe love to hear from our listeners, so feel free to send us message















