101.7 FM
88.1HD2
 8.85 c
8.85 c101.7 FM
88.1HD2
Health Canada approves AstraZeneca's COVID-19 vaccine
BY , Feb 26, 2021 4:18 PM - REPORT AN ERROR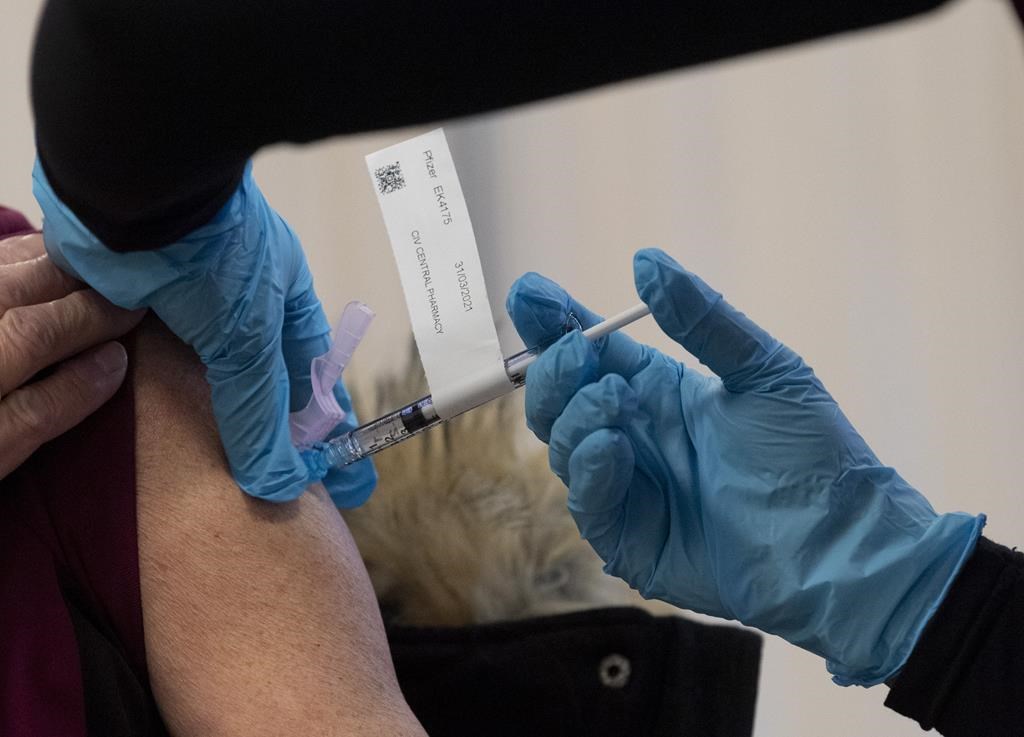
A COVID-19 vaccine is administered to a personal support worker at the Ottawa Hospital, Tuesday, December 15, 2020 in Ottawa. THE CANADIAN PRESS/Adrian Wyld
Health Canada has approved the COVID-19 vaccine from AstraZeneca, the third to be given the green light for national use.
Canada has pre-ordered 20 million doses of the AstraZeneca vaccine, which was co-developed by researchers at the University of Oxford.
It will also receive up to 1.9 million doses of the AstraZeneca vaccine through the global vaccine-sharing initiative known as COVAX by the end of June.
Vaccines produced by Pfizer-BioNTech and Moderna had already been approved by Health Canada.
Approximately 1.7 million doses of those formulas have been administered in Canada.
Health Canada senior medical advisor Doctor Supriya Sharma says, unlike the Pfizer-BioNTech and Moderna vaccines, the AstraZeneca vaccine does not have to be stored at super cold temperatures.
Health Canada continues to review two other vaccines, from Johnson and Johnson and Novavax.
Share on
Related News
Sign up for the newsletter
We'll deliver best of entertainment right into your inboxWe love to hear from our listeners, so feel free to send us message